Biofuel Production through Catalytic Pyrolysis of Biomass

Purchased from Gettyimages. Copyright.
Pyrolysis: A Promising yet Challenging Process
Energy is a vital input for production and economic growth. In today’s carbon-constrained world, renewable energy carrier development is a key component of strategies adopted to tackle ever-growing energy needs while meeting overarching greenhouse gas emission reduction goals. Biofuels continue to grow as a share of Canada’s total energy supply, but major progress is still needed to deploy bioenergy from biomass conversion [1]. This article emphasizes pyrolysis, an efficient and environmentally attractive route for producing biofuels having the potential to replace fossil hydrocarbons in boilers and engines. Fundamental challenges must, however, be addressed to elucidate the physicochemical mechanisms present during biomass pyrolysis while assessing the main features of the so-generated products. These critical requisites are currently being addressed at ÉTS, where research exploring novel production routes (including catalytic ones) for pyrolysis fuels are being conducted.
Catalytic Pyrolysis of Biomass
Lignocellulosic biomass is composed of three main polymeric constituents: hemicellulose, cellulose and lignin (cf. Fig. 1). Pyrolysis consists in decomposing these biopolymers through heating under an inert atmosphere. A variety of products formed in three states are then released, including a carbon-rich solid residue called char, a condensable vapor fraction composed of a mixture of water and organic species, and a non-condensable gaseous phase. Pyrolysis products obtained directly from raw biomass usually have certain disadvantages due to their high oxygen content, leading to high corrosiveness and a low heating value. To address this, an interesting option is to implement a catalytic treatment of biomass in order to optimize selectivity and remove oxygenated groups to produce upgraded pyrolysis products.
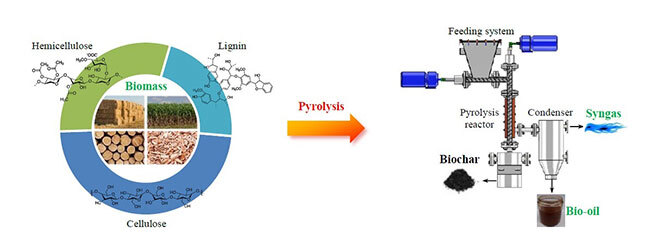
Figure 1: From biomass to biofuels
Among the catalysts currently considered, specific attention is devoted to alkali and alkaline earth metals (AAEMs) for their low toxicity, affordability and catalytic efficiency. Our recent literature review, however, highlighted the need for further research based on a systematic comparison of the impact of AAEM additives added to the same feedstocks under the same conditions, to determine their respective catalytic efficiency, notably, from a kinetic perspective [2].
Studying the Impact of AAEMs on Pyrolysis Kinetics
Fig. 2 shows the methodology used to study the AAEM-catalyzed pyrolysis of biomass. The AAEM catalysts are added to feedstocks through wet impregnation. Thermogravimetric analyses (TGA) then measure the sample mass loss as a function of the temperature, which reflects the bond breaking phenomena occurring during pyrolysis. Comparing the mass-loss profiles measured with raw and impregnated biomass then allows assessing the relative impact of the catalyst on the biopolymer decomposition. Finally, the results can be kinetically modeled using various approaches—model-fitting, iso-conversional models, global reaction schemes, etc.—to infer rate constant parameters suitable to reproduce measured data. Thus, insightful information on the reaction mechanisms involved and on the catalytic effect induced by the added AAEMS can be derived.
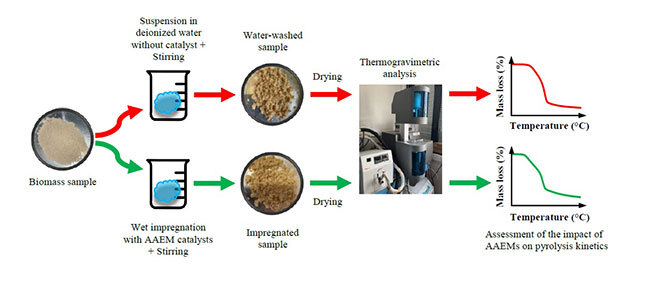
Figure 2: Methodology used to study the kinetics of AAEM-catalyzed pyrolysis of biomass
A Less Energy-Intensive Process
When analyzing the catalytic pyrolysis of beech wood and corncob impregnated with six additives containing alkali and alkaline earth metals—namely Na2CO3, NaOH, NaCl, KCl, CaCl2 and MgCl2—it was found that alkaline earth metals were more effective than alkali metals in fostering biomass decomposition at low temperatures [3]. This has been traced to the strong affinity of the former to oxygenated groups present in biopolymers and to the ability of these metallic ions to promote dehydration reactions, together with the depolymerization of hemicellulose, among others. The basic character of the catalysts was also demonstrated to be an important factor influencing the pyrolysis process. Actually, the higher the basicity of the additive, the higher its efficiency, which may be related to the strong deoxygenation capacity of basic catalysts. However, CaCl2 and MgCl2 were shown to promote repolymerization reactions, leading to increased char formation when used at high loads. This led to the conclusion that the lower the catalyst concentration, the more optimal the biomass use.
Regarding kinetic analysis, the modeling of measured data led to infer rate-constant parameters that allowed simulating the evolution of the fuel conversion degree (α) as a function of the temperature (Т) which typically follows Arrhenius-like equations:
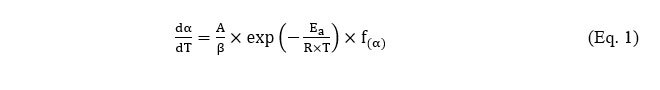
where А is the frequency factor, Ea denotes the activation energy—which reflects the minimum energy required for a reaction to occur—R corresponds to the universal gas constant, while f(α) is the differential reaction model. Obtained results highlighted significant decreases of the activation energies when impregnating a woody biomass with AAEM chlorides, thus corroborating the existence of catalytic effects shifting the decomposition process to lower temperatures [4]. This is an interesting finding as the lower the pyrolysis temperature, the less the energy required for biomass processing.
A survey of the speculated pathways accounting for the impact of AAEMs on the thermal degradation of biomass finally showed that the enhanced cleavage of chemical bonds in biomass and the promotion of char formation reactions were two major routes likely to explain the observed trends.
Conclusion
The research conducted at ÉTS provided insights contributing to a better understanding of the catalytic effect of different AAEM compounds. This work helped to elucidate the reaction and kinetic mechanisms at play during the AAEM-catalyzed pyrolysis of biomass. In the long term, this research will help identify the most suited catalysts and operating conditions in order to efficiently produce carbon-neutral biofuels derived from the conversion of non-edible resources, thus contributing in supporting the transition towards a low carbon footprint energy future.
Additional Information
For more information on this research topic, please read the following paper:
Wang, W.; Lemaire, R.; Bensakhria, A.; Luart, D. Review on the Catalytic Effects of Alkali and Alkaline Earth Metals (AAEMs) Including Sodium, Potassium, Calcium and Magnesium on the Pyrolysis of Lignocellulosic Biomass and on the Co-Pyrolysis of Coal with Biomass. Journal of Analytical and Applied Pyrolysis 2022, 163, 105479. https://doi.org/10.1016/j.jaap.2022.105479



