80% of greenhouse gas emissions come from fossil fuel combustion and industrial processes. The shift to renewable energies is therefore inescapable in our efforts to limit climate change. However, this transition involves a number of challenges. The reliability of renewable energy sources is irregular at best, hence the need for energy conversion and storage systems. Our research team is dedicated to designing and manufacturing innovative nanomaterials for batteries, fuel cells and hydrogen production systems. Our goal is to address the critical and long-standing challenges of these clean energy devices (e.g., enhancing performance, improving reliability, and reducing cost) and promote their integration into energy systems.
Batteries Are a Must
Surplus energy generated by renewable sources, such as wind or solar power, could be stored in batteries for later use. Batteries can play a vital role in the energy transition. Using various nanomaterials, our research team is developing various advanced electrodes, electrolytes, and their interfaces to improve different types of batteries to make them more efficient and less polluting
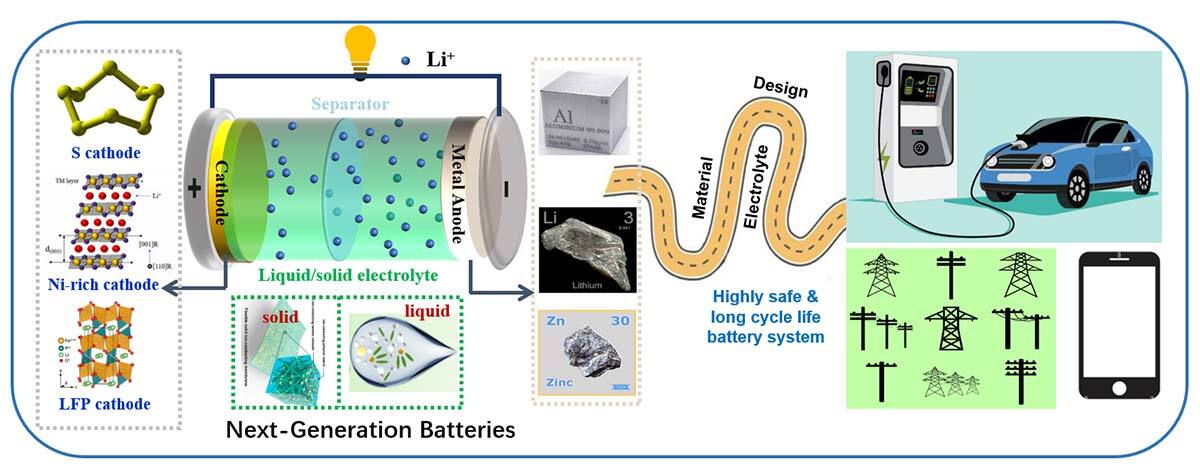
We are working, among other things, on developing long-lasting, efficient and safe lithium, zinc, aluminum, and all-solid-state batteries. These batteries are designed for applications in energy storage and power supply, as well as flexible and portable electronics.
Batteries, while necessary in ensuring the transition from fossil fuels to clean energies, are not perfect storage systems. They typically consist of rare, expensive and sometimes toxic metals, and are challenging to recycle. We must improve battery technology and in the meantime continue our research and develop even cleaner technologies, such as hydrogen.
Hydrogen – 100% Clean Technology
Electrolysis is a process that separates water molecules into oxygen and hydrogen. The hydrogen thus formed stores clean energy, which can be used in fuel cells and energy carrier for industry use.
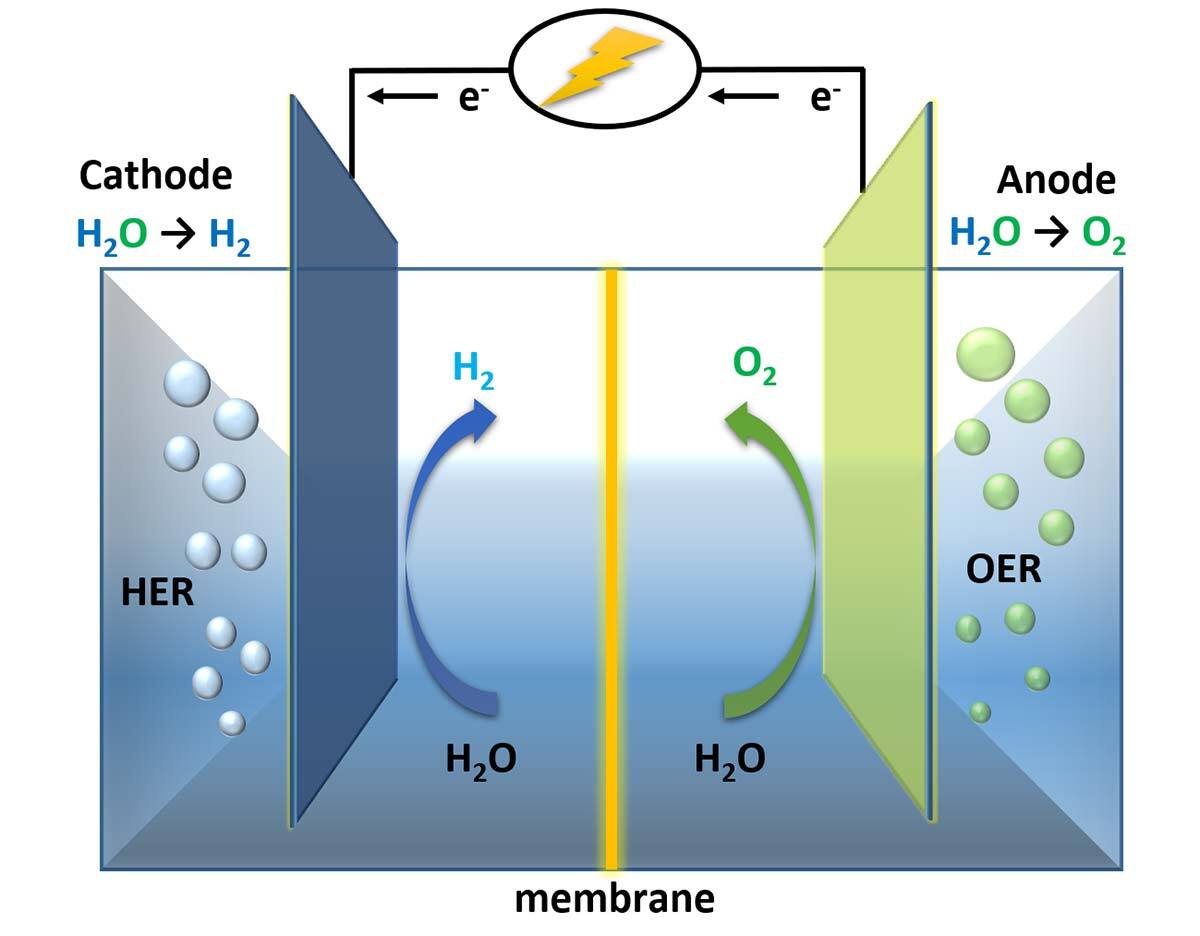
A hydrogen fuel cell represents a 100% clean technology since, in addition to hydrogen, it consumes oxygen from air to form essentially pure water. It therefore generates no toxic products or greenhouse gases. In the Quebec context, since hydroelectric power generation is based on peak demand, it would be beneficial to use the unconsumed energy of off-peak periods to produce hydrogen, and then use it in an energy conversion system to produce power on demand.
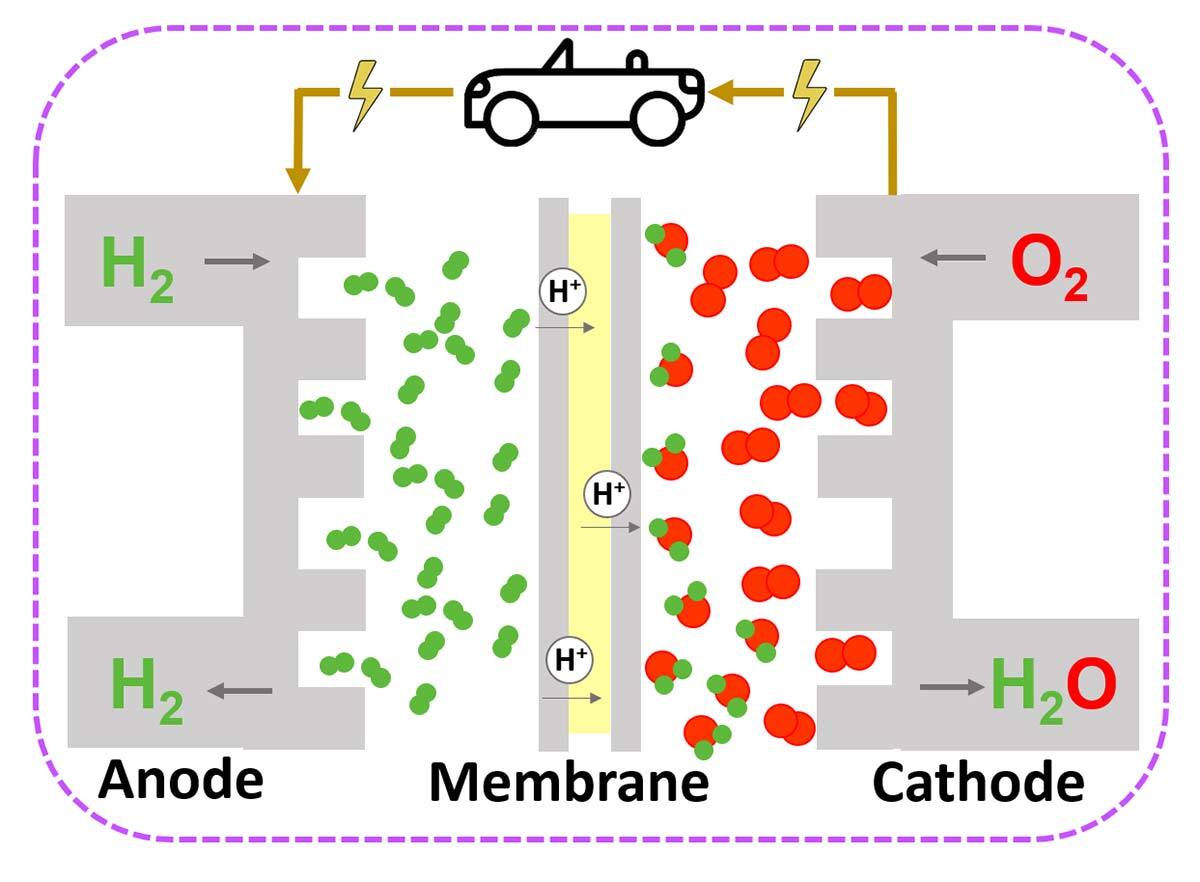
It’s also worth mentioning that hydrogen fuel cells are extremely safe. Hydrogen, an ultra-light gas, tends to dissipate into the atmosphere in open environments rather than forming concentrations. In addition, research is ongoing to develop various devices preventing hydrogen escaping from tanks, making this technology safe even in closed environments such as tunnels.
Conclusion
Energy transition plays a crucial role in our fight against climate change. We must develop new carbon-neutral and environmentally friendly technologies to take full advantage of renewable energies.